
A Great-winged Petrel incubates an egg in its burrow
NOTE: This post is the ninth in an occasional series on the website of the Marion Mouse-Free Project that features breeding seabirds of Marion Island which are being attacked by introduced House Mice, or are considered to be at risk to them.
Stefan Schoombie of the University of Cape Town’s Centre for Statistics in Ecology, the Environment and Conservation writes on working with the winter-breeding Great-winged Petrel Pterodroma macroptera during his several long sojourns on South Africa’s sub-Antarctic possession in the southern Indian Ocean.

With breeding Sooty Albatrosses looking on, Stefan Schoombie abseils down a coastal cliff on Marion Island
I have had the privilege of spending a considerable amount of time on Marion Island over the past nine years. Most of my research involved albatrosses and large petrels, but in 2019 I worked on a project focusing on the smaller burrowing petrels. There are several species of burrow-nesting petrels breeding on Marion Island. Burrow-nesting birds are something we are not often familiar with on the mainland, but they are a common occurrence on sub-Antarctic islands, where millions of birds tunnel underground to escape the harsh weather and the larger seabirds that prey on them. Studying burrowing birds can be challenging, but all the more rewarding/ as we have the privilege of seeing these special birds from close up.
On Marion Island we monitored a subset of Great-winged Petrel nests, following their progress throughout the breeding season. These birds can dig long tunnels, stretching several metres, and we often had to install inspection hatches to enable monitoring of the nest contents. However, some of the burrows can be very shallow, allowing a unique view of these beautifully sleek birds.

A view from a burrowscope – a Great-winged Petrel adult and its chick in their nest
Studying birds that nest several metres underground has its challenges, but we now have tools accessible to us enabling more efficient monitoring of their breeding habits. The above image was recorded by a small camera attached to a light and mounted on a flexible tube, known as a “burrowscope”. These cameras allow us to monitor the birds more efficiently, as we can review images afterwards. In 2016, during one of these nest checks, we found several mice attacking a very small Great-winged Petrel chick. Unfortunately, the bird died shortly after discovering the incident, but by documenting the attack we could add Great-winged Petrels to the list of seabirds affected by mice on Marion Island.

Burrowscope photograph of a House Mouse feeding on a two-day-old Great-winged Petrel chick on Marion Island, the chick did not survive, from a video by Stefan Schoombie (see Dilley et al. 2018 for details of the attack)

Night birding – by shining a bright light into the sky, nocturnal seabirds are caught at their breeding sites as they become dazzled and land
Burrowing birds are often nocturnal, because predators, such as Brown Skuas Stercorarius antarcticus, can easily catch them during the day when they arrive at their nests. Once fully dark, the sky is filled with thousands of birds echoing in a cacophony of different calls and a wind-still night in the breeding season is a marvellous experience on a sub-Antarctic island. On misty nights we could catch smaller petrels by shining a bright light into the sky, disorienting the birds and resulting in them crash-landing, usually onto soft vegetation, before we caught them in a net. I did a lot of this work in the company of my wife, Janine Schoombie, and here she is in the image above attracting a bird in the torch light. Great-winged Petrels are among only a couple of species that breed during the winter months on Marion Island, so working on them is often challenging as winter nights can be pretty cold in the sub-Antarctic.

Ready to fledge. A Great-Winged Petrel chick that has lost nearly all its down feathers, with the Marion Island base in the background
Photographs by Stefan Schoombie
In 2019 I worked on a project with the Marine Apex Predator Research Unit (MAPRU) at South Africa’s Nelson Mandela University, when we studied a range of burrowing petrels that breed on Marion Island. These included Great-winged Petrels and I took samples and measurements from a number of adults and chicks. These data will contribute to our understanding of the species’ foraging habits, and also give an insight into the amount of microplastics that are found in their diet. During this work I had the privilege to handle many birds, giving me a unique opportunity to view them up-close, something for which I will always be grateful.
The impact of mice on Great-winged Petrels at Marion emphasizes the importance of the Mouse-Free Marion Project in eradicating the sole remaining introduced mammal on the island, thus markedly improving their conservation status. I wish the project every success!
Read Stefan’s MFM News post on Marion Island’s globally Endangered Sooty Albatrosses Phoebetria fusca here.
Selected Publications:
Cooper, J. & Fourie, A. 1991. Improved breeding success of Great-winged Petrels Pterodroma macroptera following control of feral cats Felis catus at subantarctic Marion Island. Bird Conservation International 1: 171-175.
Cooper, J. & Klages, N.T.W. 2009. The winter diet of the Great-winged Petrel Pterodroma macroptera at sub-Antarctic Marion Island in 1991. Marine Ornithology 37: 261-263.
Cooper, J. Marais, A.V.N., Bloomer, J.P. & Bester, M.N. 1995. A success story: breeding of burrowing petrels (Procellaridae) before and after eradication of feral cats Felis catus at sub-Antarctic Marion Island. Marine Ornithology 23: 33-37.
Dilley, B.J., Davies, D., Stevens, K., Schoombie, S., Schoombie, J. & Ryan, P.G. 2019. Burrow wars and sinister behaviour among burrow-nesting petrels at sub-Antarctic Marion Island. Ardea 107: 97-102.
Dilley, B.J., Schoombie, S., Stevens, K., Davies, D., Perold, V., Osborne, A., Schoombie, J., Brink, C.W., Carpenter-Kling, T. & Ryan, P.G. 2018. Mouse predation affects breeding success of burrow-nesting petrels at sub-Antarctic Marion Island. Antarctic Science 30: 93-104.
Schramm, M. 1983. The breeding biologies of the petrels Pterodroma macroptera, P. brevirostris and P. mollis at Marion Island. Emu 83: 75-81.
Schramm, M. 1986. The diet of chicks of Greatwinged, Kerguelen and Softplumaged Petrels at the Prince Edward Islands. Ostrich 57: 9-15.
Schramm, M. 1986. Burrow densities and nest site preferences of petrels (Procellariidae) at the Prince Edward Islands. Polar Biology 6: 63-70.
Stefan Schoombie, Centre for Statistics in Ecology, the Environment and Conservation, University of Cape Town, Rondebosch, South Africa, 27 October 2022
 Displaying Amsterdam Albatrosses; photograph by Romain Buenadicha
Displaying Amsterdam Albatrosses; photograph by Romain Buenadicha
 English
English  Français
Français  Español
Español 









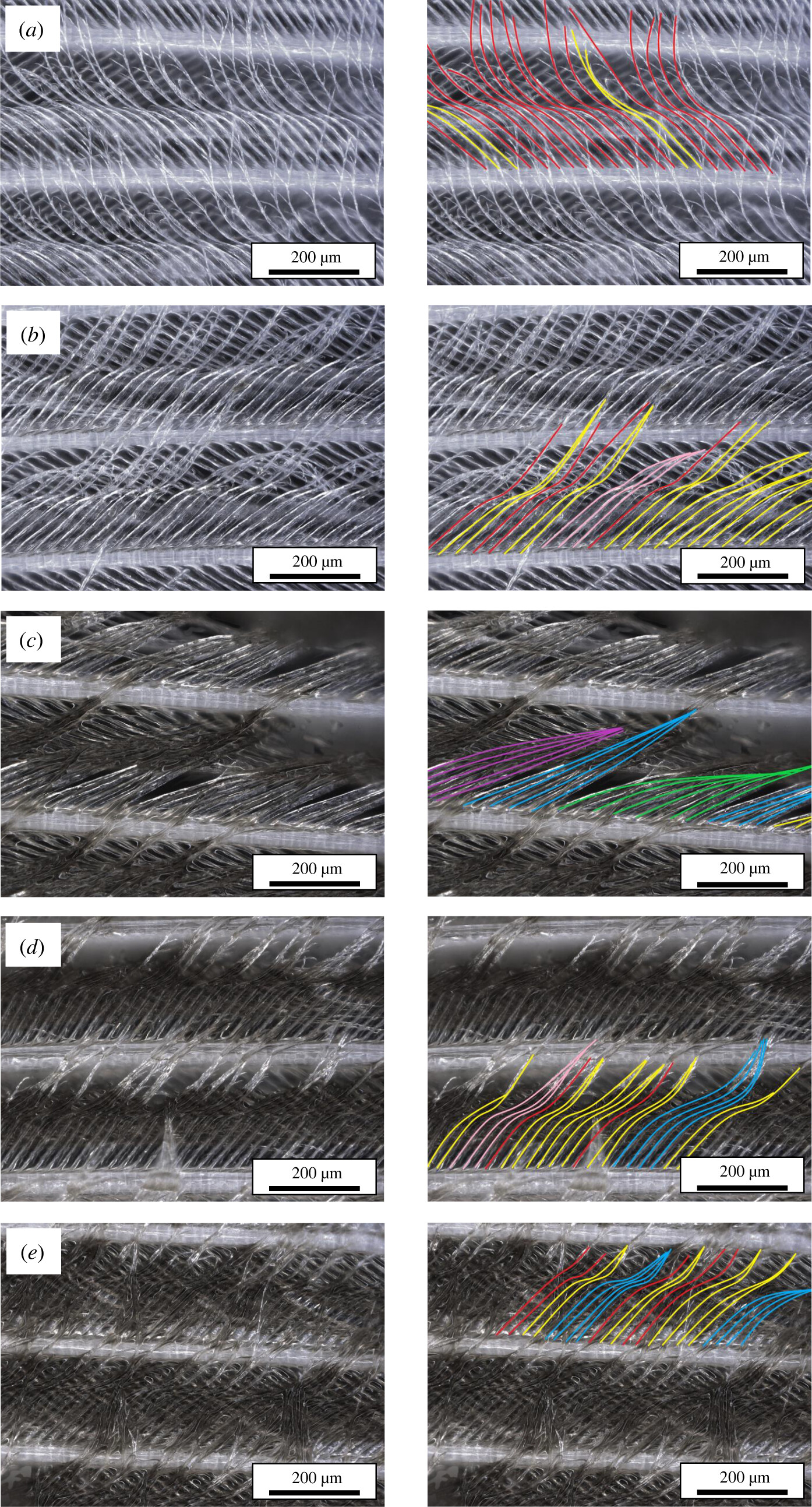 An image from the paper: Figure 4. Amalgamation annotation of Manx shearwater feathers. Left: the distribution of barbules along the barb at increasing levels of oil treatment. Oil treatments are (a) control, (b) trace colour sheen – 0.1 µm, (c) dark colour sheen – 3 µm, (d) standard slick – 25 µm, and (e) severe slick – 75 µm, (
An image from the paper: Figure 4. Amalgamation annotation of Manx shearwater feathers. Left: the distribution of barbules along the barb at increasing levels of oil treatment. Oil treatments are (a) control, (b) trace colour sheen – 0.1 µm, (c) dark colour sheen – 3 µm, (d) standard slick – 25 µm, and (e) severe slick – 75 µm, (